Desmoid Tumors
People living with rare tumors need more options – right now. Let’s unleash
the possibilities.
Desmoid tumors are rare, aggressive soft-tissue tumors characterized by locally invasive growth, significant morbidity, and a high rate of recurrence. They are often debilitating and disfiguring and can invade surrounding tissues and structures.
Desmoid tumors do not metastasize (spread from one body part to another), however, they are known for having unpredictable behavior and treatment approaches may vary. These tumors can cause severe pain and morbidity resulting from disfigurement, vascular constriction, and loss of physical function. When aggressive and near or involving vital structures, desmoid tumors can be life-threatening.
Watch Kevin's desmoid tumor story
Where do desmoid tumors grow?
While desmoid tumors can arise in any part of the body, the most common sites are the intra-abdominal and abdominal wall, lower extremities (hip, thigh, leg, and foot), thoracic/chest wall areas, upper extremities (arm, forearm, and hand), and the head and neck.
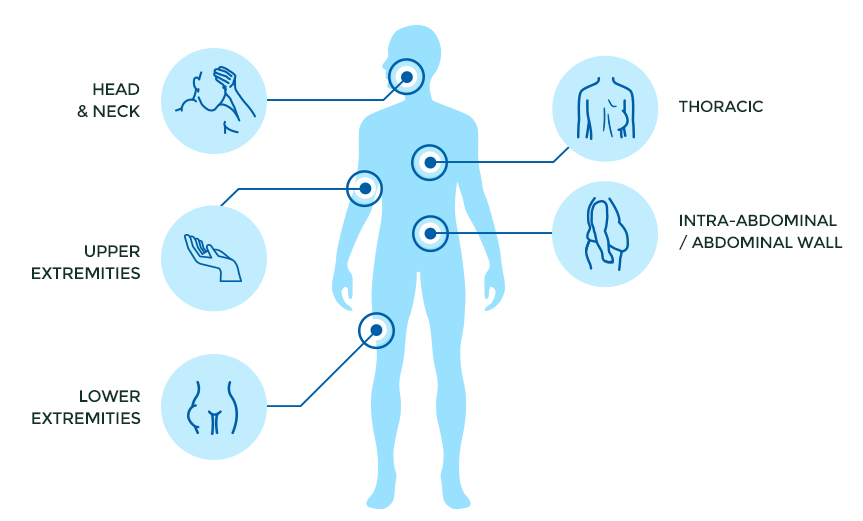
- Head & Neck (8%)
- Upper Extremities (14%)
- Lower Extremities (16%)
- Thoracic/Chest Wall (15%)
- Intra-abdominal (20%)
- Abdominal Wall (16%)
- Other locations (11%)
Source: Constantinidou, Anastasia & Scurr, Michelle & Judson, Ian & Litchman, Charisse. (2012). Clinical Presentation of Desmoid Tumors. 10.1007/978-94-007-1685-8_2.
Who gets desmoid tumors?
While desmoid tumors can occur in people of almost any age, they are most commonly diagnosed in adults between 20-44 years of age. Women are also two to three times more likely to be diagnosed with a desmoid tumor than men. It is estimated that there are 1,000 to 1,650 new cases diagnosed each year in the United States with a population-proportionate incidence in other countries.
What are the symptoms?
The severity of a desmoid tumor generally depends on the size and location of the tumor and the aggressiveness of its growth pattern. Typically, if the tumor is in the extremities or on the chest wall, most patients will notice a lump as the initial clinical presentation. Desmoid tumors and its “tendril-like” growths may also wrap around nearby structures, causing pain (often neuropathic, which is a nerve problem that causes pain), changes to body shape and physical function, limited range of motion, sleep changes, shortness of breath, and fatigue.
Are there treatment options?
OGSIVEO (nirogacestat) is approved in the U.S. for the treatment of adult patients with progressing desmoid tumors who require systemic treatment. Historically, desmoid tumors were treated with surgical resection, but this approach has become less commonly recommended in recent treatment guidelines due to the documented high rates of tumor recurrence after surgery.
Please see important safety information including link to full Prescribing Information for OGSIVEO at the bottom of this page.
How are desmoid tumors diagnosed?
The definitive diagnosis of desmoid tumors is typically made with a core-needle biopsy, a procedure that removes tissue for pathology testing. Immunohistochemistry and genetic testing are recommended to make the diagnosis. Often it is helpful for an expert soft-tissue pathologist to confirm the desmoid tumor diagnosis to aid in an accurate and timely diagnosis.
What are the causes?
While exact causes for the development of desmoid tumors are unknown, hormone-dependent signaling, mechanical stresses, trauma, and healing processes have been proposed as possible factors involved in tumor development and growth. Desmoid tumors may be caused by genetic mutations in the DNA where fibroblasts (a cell that is critical to keeping the entire structure of our body intact) behave abnormally and turn into desmoid tumors. Other desmoid tumors are caused by mutations in a gene that causes Familial Adenomatous Polyposis (FAP). People with FAP are at high risk of developing abdominal desmoid tumors.
Looking for more information?
Additional information from SpringWorks can be found at desmoidtumors.com. You may also find it helpful to reach out to the organizations listed below for information or support.*
* The organizations listed are independent of SpringWorks Therapeutics.
Important Safety Information about OGSIVEO
WARNINGS AND PRECAUTIONS
- Diarrhea: Diarrhea, sometimes severe, can occur in patients treated with OGSIVEO. Diarrhea occurred in 84% of patients treated with OGSIVEO, and included Grade 3 events in 16% of patients. Median time to first diarrhea event was 9 days (range: 2 to 434 days). Monitor patients and manage using antidiarrheal medications. Modify dose as recommended.
- Ovarian Toxicity: Female reproductive function and fertility may be impaired in patients treated with OGSIVEO. Impact on fertility may depend on factors like duration of therapy and state of gonadal function at time of treatment. Long-term effects of OGSIVEO on fertility have not been established. Advise patients on the potential risks for ovarian toxicity before initiating treatment. Monitor patients for changes in menstrual cycle regularity or the development of symptoms of estrogen deficiency, including hot flashes, night sweats, and vaginal dryness.
- Hepatotoxicity: ALT or AST elevations occurred in 30% and 33% of patients, respectively. Grade 3 ALT or AST elevations (>5 × ULN) occurred in 6% and 2.9% of patients. Monitor liver function tests regularly and modify dose as recommended.
- Non-Melanoma Skin Cancers: New cutaneous squamous cell carcinoma and basal cell carcinoma occurred in 2.9% and 1.4% of patients, respectively. Perform dermatologic evaluations prior to initiation of OGSIVEO and routinely during treatment.
- Electrolyte Abnormalities: Decreased phosphate (65%) and potassium (22%) occurred in OGSIVEO-treated patients. Phosphate <2 mg/dL occurred in 20% of patients. Grade 3 decreased potassium occurred in 1.4% of patients. Monitor phosphate and potassium levels regularly and supplement as necessary. Modify dose as recommended.
- Embryo-Fetal Toxicity: OGSIVEO can cause fetal harm when administered to pregnant women. Oral administration of nirogacestat to pregnant rats during the period of organogenesis resulted in embryo-fetal toxicity and death at maternal exposures below human exposure at the recommended dose of 150 mg twice daily. Advise pregnant women of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose.
ADVERSE REACTIONS
- The most common (≥15%) adverse reactions were diarrhea (84%), ovarian toxicity (75% in the 36 females of reproductive potential), rash (68%), nausea (54%), fatigue (54%), stomatitis (39%), headache (30%), abdominal pain (22%), cough (20%), alopecia (19%), upper respiratory tract infection (17%), and dyspnea (16%).
- Serious adverse reactions occurred in 20% of patients who received OGSIVEO. Serious adverse reactions occurring in ≥2% of patients were ovarian toxicity (4%).
- The most common laboratory abnormalities (≥15%) were decreased phosphate, increased urine glucose, increased urine protein, increased AST, increased ALT, and decreased potassium.
DRUG INTERACTIONS
- CYP3A Inhibitors and Inducers: Avoid concomitant use with strong or moderate CYP3A inhibitors (including grapefruit products, Seville oranges, and starfruit) and strong or moderate CYP3A inducers.
- Gastric Acid Reducing Agents: Avoid concomitant use with proton pump inhibitors and H2 blockers. If concomitant use cannot be avoided, OGSIVEO can be staggered with antacids (e.g., administer OGSIVEO 2 hours before or 2 hours after antacid use).
- Consult the full Prescribing Information prior to and during treatment for important drug interactions.
USE IN SPECIFIC POPULATIONS
- Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with OGSIVEO and for 1 week after the last dose.
Please see accompanying full Prescribing Information.





