Nirogacestat (Gamma Secretase Inhibitor)
We measure ourselves by the impact we have on people with rare diseases.
Let’s go big.
OGSIVEO (nirogacestat) is an oral, selective, small molecule gamma secretase inhibitor (GSI) that is approved in the U.S. for use in adults with progressing desmoid tumors who require systemic treatment.
Nirogacestat is also in Phase 2 clinical development for adult patients with ovarian granulosa cell tumors and is being evaluated as part of several B-cell maturation agent (BCMA) combination therapy regimens in collaboration with industry and academia.
Nirogacestat (gamma secretase inhibitor)
Gamma secretase cleaves multiple transmembrane proteins, including Notch, that are believed to play a role in activating pathways that contribute to growth of desmoid and ovarian granulosa cell tumors.
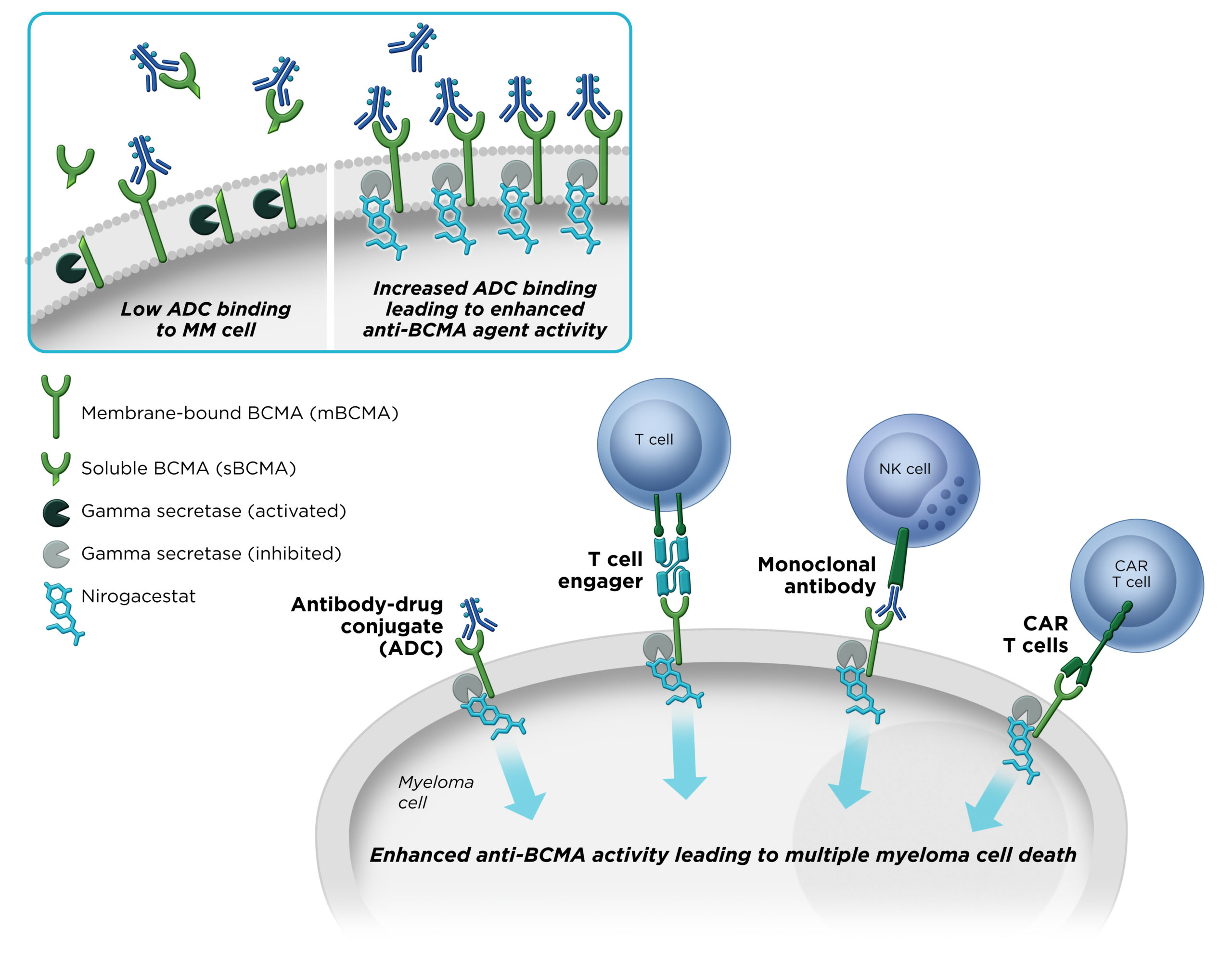
Gamma secretase has been shown to cleave BCMA, a therapeutic target that is specifically expressed on multiple myeloma cells. We believe that by inhibiting gamma secretase with nirogacestat, membrane-bound BCMA can be preserved on the surface of myeloma cells, thereby increasing target density while simultaneously reducing levels of soluble BCMA, which may interfere with the activity BCMA-directed therapies. Combining nirogacestat with BCMA-targeted therapies may increase their activity and improve outcomes for multiple myeloma patients. SpringWorks is advancing nirogacestat as a potential cornerstone of multiple myeloma combination therapy across modalities in collaboration with industry-leading BCMA therapy developers.
OGSIVEO (nirogacestat) for desmoid tumors
OGSIVEO (nirogacestat) is approved in the U.S. for the treatment of adult patients with progressing desmoid tumors who require systemic treatment. The approval was based on a global, randomized, double-blind, placebo-controlled Phase 3 clinical trial (the DeFi trial), which evaluated the efficacy, safety, and tolerability of nirogacestat in adult patients with progressing desmoid tumors.
In September 2022, positive data from the Phase 3 DeFi trial were presented at the ESMO Congress 2022, and in March 2023, data from the trial were published in the New England Journal of Medicine (Gounder M, Ratan R, Alcindor T, et al. Nirogacestat, a gamma-secretase inhibitor for desmoid tumors. N Engl J Med. 2023;388(10):898-912).
Please see important safety information including link to full Prescribing Information for OGSIVEO at the bottom of this page.
Nirogacestat in ovarian granulosa cell tumors
Ovarian granulosa cell tumors (OvGCT) account for approximately 5% of all ovarian cancers and are the most common subtype of ovarian sex cord stromal tumors. It is estimated that there are 1,500 to 2,000 new cases diagnosed per year in the U.S., and an estimated prevalence of approximately 10,000-15,000 patients with OvGCT in the U.S. There are no FDA-approved therapies for patients with OvGCT. SpringWorks is conducting a Phase 2 trial to evaluate the efficacy, tolerability, safety, and pharmacokinetics of nirogacestat for the treatment of patients with recurrent OvGCT (NCT05348356) .
The safety and efficacy of nirogacestat in OvGCT has not been established.
Nirogacestat in multiple myeloma
Multiple myeloma is the second most common hematologic malignancy in the U.S., accounting for approximately 10 percent of all hematologic cancers. There are approximately 130,000 patients in the U.S. living with multiple myeloma.
B-cell maturation antigen (BCMA) is a cell surface protein that is highly and specifically expressed on multiple myeloma cells. BCMA has emerged as a promising target in multiple myeloma across several therapeutic modalities. Gamma secretase directly cleaves membrane-bound BCMA.
We believe that by inhibiting gamma secretase with nirogacestat, membrane-bound BCMA can be preserved, thereby increasing target density while simultaneously reducing levels of soluble BCMA, which may interfere with BCMA-directed therapies. Together, these mechanisms combine to potentially enhance the activity of BCMA-directed therapies and improve outcomes for multiple myeloma patients.
Nirogacestat’s ability to significantly enhance the activity of BCMA-directed therapies has been observed in several preclinical models of human multiple myeloma.
In preclinical models of multiple myeloma, nirogacestat significantly enhanced the activity of BCMA-directed therapies across therapeutic modalities.
Nirogacestat is being investigated in combination with BCMA therapies
Ongoing collaborations with industry-leading developers of BCMA-targeted therapies
The safety and efficacy of nirogacestat, alone or in combination with other agents, for the treatment of multiple myeloma has not been established.
Important Safety Information about OGSIVEO
WARNINGS AND PRECAUTIONS
- Diarrhea: Diarrhea, sometimes severe, can occur in patients treated with OGSIVEO. Diarrhea occurred in 84% of patients treated with OGSIVEO, and included Grade 3 events in 16% of patients. Median time to first diarrhea event was 9 days (range: 2 to 434 days). Monitor patients and manage using antidiarrheal medications. Modify dose as recommended.
- Ovarian Toxicity: Female reproductive function and fertility may be impaired in patients treated with OGSIVEO. Impact on fertility may depend on factors like duration of therapy and state of gonadal function at time of treatment. Long-term effects of OGSIVEO on fertility have not been established. Advise patients on the potential risks for ovarian toxicity before initiating treatment. Monitor patients for changes in menstrual cycle regularity or the development of symptoms of estrogen deficiency, including hot flashes, night sweats, and vaginal dryness.
- Hepatotoxicity: ALT or AST elevations occurred in 30% and 33% of patients, respectively. Grade 3 ALT or AST elevations (>5 × ULN) occurred in 6% and 2.9% of patients. Monitor liver function tests regularly and modify dose as recommended.
- Non-Melanoma Skin Cancers: New cutaneous squamous cell carcinoma and basal cell carcinoma occurred in 2.9% and 1.4% of patients, respectively. Perform dermatologic evaluations prior to initiation of OGSIVEO and routinely during treatment.
- Electrolyte Abnormalities: Decreased phosphate (65%) and potassium (22%) occurred in OGSIVEO-treated patients. Phosphate <2 mg/dL occurred in 20% of patients. Grade 3 decreased potassium occurred in 1.4% of patients. Monitor phosphate and potassium levels regularly and supplement as necessary. Modify dose as recommended.
- Embryo-Fetal Toxicity: OGSIVEO can cause fetal harm when administered to pregnant women. Oral administration of nirogacestat to pregnant rats during the period of organogenesis resulted in embryo-fetal toxicity and death at maternal exposures below human exposure at the recommended dose of 150 mg twice daily. Advise pregnant women of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose.
ADVERSE REACTIONS
- The most common (≥15%) adverse reactions were diarrhea (84%), ovarian toxicity (75% in the 36 females of reproductive potential), rash (68%), nausea (54%), fatigue (54%), stomatitis (39%), headache (30%), abdominal pain (22%), cough (20%), alopecia (19%), upper respiratory tract infection (17%), and dyspnea (16%).
- Serious adverse reactions occurred in 20% of patients who received OGSIVEO. Serious adverse reactions occurring in ≥2% of patients were ovarian toxicity (4%).
- The most common laboratory abnormalities (≥15%) were decreased phosphate, increased urine glucose, increased urine protein, increased AST, increased ALT, and decreased potassium.
DRUG INTERACTIONS
- CYP3A Inhibitors and Inducers: Avoid concomitant use with strong or moderate CYP3A inhibitors (including grapefruit products, Seville oranges, and starfruit) and strong or moderate CYP3A inducers.
- Gastric Acid Reducing Agents: Avoid concomitant use with proton pump inhibitors and H2 blockers. If concomitant use cannot be avoided, OGSIVEO can be staggered with antacids (e.g., administer OGSIVEO 2 hours before or 2 hours after antacid use).
- Consult the full Prescribing Information prior to and during treatment for important drug interactions.
USE IN SPECIFIC POPULATIONS
- Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with OGSIVEO and for 1 week after the last dose.
Please see accompanying full Prescribing Information.